To assist in the battle against COVID-19, a number of University research projects in progress since early spring seek to develop new technology molded to the needs of medical personnel, COVID-19 patients and testing sites.
Two of these projects are aimed specifically at preserving lung function and diagnosing COVID-19 through a single breath. The “PufferFish” ventilator and “Bubbler” testing kit, respectively, along with a plethora of other projects, were funded by the COVID-19 Research Seed Fund, as The Herald previously reported.
Breathe in: PufferFish Ventilator from PezGlobo
[caption id="attachment_2840092" align="aligncenter" width="650" caption="Researchers at Brown, Stanford University and the University of Utah have collaborated on a ventilator that can be rebuilt by other professionals and provides a display that facilitates the interaction between man and machine."]
“It’s ... breathtaking,” the researchers of the BrunO2 research group tweeted in March as they watched an early prototype of their ventilator, an entanglement of tubes and wires, inflate and deflate a balloon. The BrunO2 team, a small group of University faculty and students, built the device with the hope that one day it would fill a COVID-19 patient’s lungs with air. In the past few months, this prototype — a contestant in the Code Life Ventilator Challenge — has transformed into a new device, created by merging minds across campus and the world.
Now inflating lungs like its namesake, the “PufferFish” ventilator is the product of PezGlobo (meaning pufferfish in Spanish) — a collaboration that has expanded to also include Stanford University, the University of Utah and industrial and financial companies. The group’s website launched July 10.
Earlier this spring, the anticipated surge in COVID-19 cases did not reach Rhode Island, relieving the threat of a ventilator shortage for the time being, according to Co-Principal Investigator and Assistant Professor of Engineering Daniel Harris.
But even as the immediate local need for ventilators abated, the researchers continued deliberating, designing and tinkering.
In the event of another COVID-19 wave, the researchers expect their design to be beneficial to patients — especially because flexibility in the ventilator’s composition ensures its accessibility, Co-Principal Investigator and Assistant Professor of Engineering Jacob Rosenstein said. Using comparatively affordable and readily available components, the team hopes to also make their device a viable option in nations with fewer medical resources.
By making the ventilator’s components, building instructions and source code available worldwide, the researchers will allow healthcare professionals to build the ventilators themselves. In this way, their work may elevate the application of open-source hardware — a concept that is still developing in the realm of medical technology.
When the pandemic was still in its infancy in the United States, Co-Principal Investigator and Professor of Engineering Roberto Zenit received a notification about the Code Life Ventilator Challenge and brought Harris, Rosenstein and several others on board. With a firm deadline approaching for the competition, they gave up their days and nights to build the machine.
But they soon realized that a project of such magnitude — with an impact that they hoped would extend beyond the confines of the competition — would demand input from future users, Harris said.
To offer these perspectives, medical students, providers and technicians have since weighed in on the project, Zenit said. Researchers from Stanford and the University of Utah, who had been collaborating on their own Vent4US ventilator, later partnered with Brown. Researchers from other nations, including Chile, Germany, India and Kenya, have also contributed, with some building and testing the ventilator at their home institutions.
Harris emphasized the importance of interdisciplinary collaboration and acknowledged the “innate creativity and curiosity with new problems” that students brought to this interinstitutional undertaking.
The design “has changed a lot, but the spirit remains the same,” he said of the latest and last ventilator model.
[caption id="attachment_2840093" align="aligncenter" width="650" caption="There are multiple means to deliver oxygen to a patient non-invasively using the ventilator."]
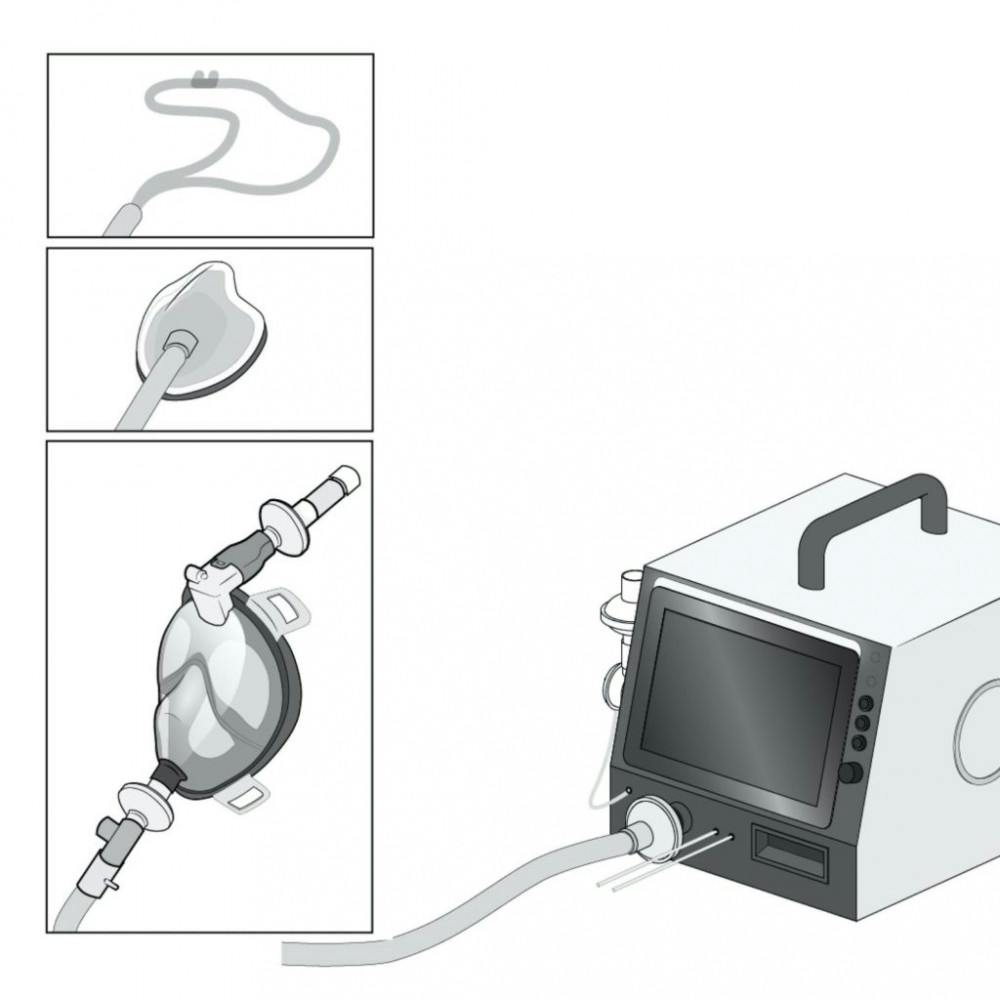
This ventilator can attach to oxygen lines in hospitals or to portable oxygen tanks and deliver oxygen through various channels, such as the mouth, nose or through a face mask. To measure and regulate air pressure delivered to a patient, the device includes sensors and valves. Its design ensures that these valves — which were designed by students — did not come in contact with the air inhaled or exhaled by a patient, allowing researchers the freedom to forgo the usual expensive and scarce sterile materials. Still, stringent certifications apply to the “medical grade tubing” that is opened and closed by the device, delivering air to the patient.
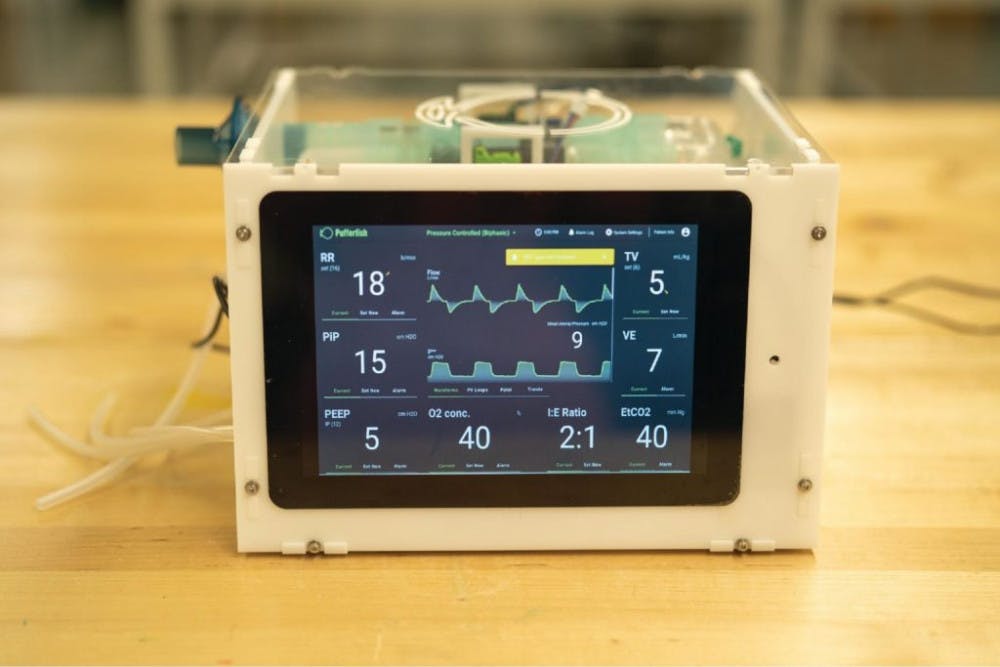
The ventilator’s real-time controls and touchscreen interface are the physician’s portal into the complicated machinery operating underneath. They are critical to functionality and ease of use in situations of urgency, Rosenstein said.
[caption id="" align="aligncenter" width="650" caption="The PufferFish ventilator features real-time controls, regulating complex machinery within."]

Above all, safety remains of utmost importance. “There’s this kind of dance in the electronic design between making it beautiful and fancy and sophisticated and also simple enough that we can promise that it’s safe,” Rosenstein said.
The researchers have been testing their device using breathing simulators and are applying for FDA Emergency Use Authorization approval.
“Everybody kind of has an attitude that this is for the common good, whether it’s somebody that needs this device today or whether it's somebody who's going to need it next year somewhere else in the world,” Rosenstien said.
“What this project has demonstrated is that (if you) put people together with a common goal to do a good deed, you can achieve amazing things very quickly,” Zenit said.
Breathe out: The Bubbler, a COVID-19 testing kit
Worldwide, limited access to testing remains one of the greatest impediments to combating COVID-19.
University genomics researchers have sought to create a COVID-19 testing technique that would expedite the current process and allow for mass surveillance testing in the future. The researchers, led by Professor of Biology Will Fairbrother, have dubbed their testing device “The Bubbler” — in reference to its design and in fond homage to Rhode Islanders’ name for a water fountain.
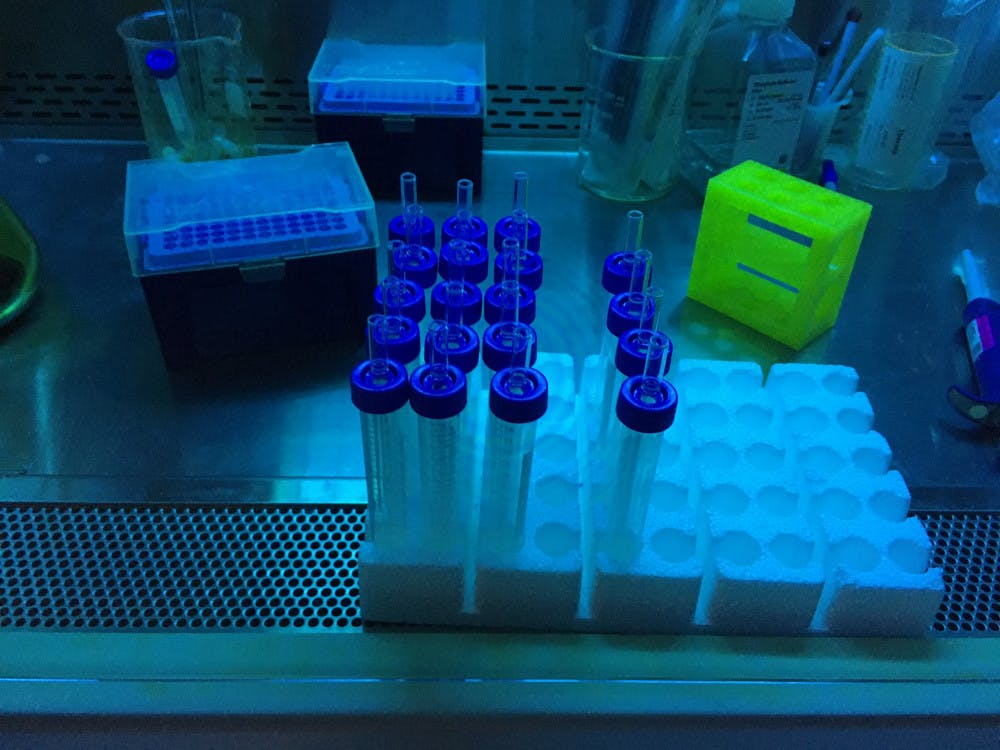
[caption id="attachment_2840086" align="aligncenter" width="650" caption="The Bubbler testing kit consists of a capped glass tube, a glass straw and a reaction solution. The person getting tested blows into the solution, exhaling any viral particles in their breath."]

The current method of COVID-19 testing involves retrieving a swab sample from the nose. This technique is not only uncomfortable but also hazardous for the technician administering the test, who risks being exposed if a COVID-19 positive patient sneezes during the process. Running the current test in the lab can take only a few hours, Fairbrother said, and people may get results back in a few days.
But what if this technique could be simplified — the first step as simple as a child blowing bubbles with a straw?
This is the principle idea behind The Bubbler testing kit, which collects samples from patients by having them blow into a clear tube of liquid through a glass straw, an action that produces bubbles.
The ease with which this test could be administered is not only safer in practice but also captures a more “critical” sample. When an infected person breathes into the straw, particles containing the viral protein — similar to those that would be released when a patient coughs or talks — are exhaled.
Alternatively, a sample could be obtained by scraping a patient’s mouth and inserting the resultant material into the tube.
Once the particles are ejected into the liquid, the solution catalyzes a chemical reaction immediately. If the tested person has COVID-19, the reverse transcriptase enzyme, a protein in the solution, makes DNA from the SARS-CoV-2’s RNA genome. This process differs from that of other tests in which this reaction takes place at a later stage.
[caption id="attachment_2840089" align="aligncenter" width="650" caption="Researchers hope COVID-19 test samples from The Bubbler could be analyzed collectively due to a primer barcoding technique that would be unique to the reaction solution in each tube."]

Even though receiving an individual test result would not necessarily be faster using this method, researchers think The Bubbler will prove more efficient than standard tests, as it allows for a large quantity of samples to be sent for simultaneous analysis, rather than analyzing the results of one patient at a time, Fairbrother said.
But for this large-scale process to work, each Bubbler would need to be linked to a specific person. The reaction in the tube requires a primer — a starter DNA molecule from which reverse transcriptase can copy any viral RNA in the solution for downstream analysis. In order to tag each sample, a portion of this sequence can be purposefully designed for each kit and thereby each patient — like a barcode.
“We’re trying to get a rapid test; we’re trying to get something that we could mail out to people,” Fairbrother said.
[caption id="attachment_2840091" align="aligncenter" width="650" caption="The Bubbler testing kit would be conveniently and neatly packaged for easy use and delivery and wide application."]

This type of test also has potential to disclose the exact genetic code that each tested person’s virus carries, which may harbor information on how the virus has evolved and its mode of transmission. It may also help diagnose other illnesses.
To gather data on The Bubbler’s effectiveness, patients tested for COVID-19 at Rhode Island Hospital have been asked to participate in the experimental testing method alongside their standard test. All personnel involved in handling the kits are taking the necessary safety precautions, including decontaminating the kit using ultraviolet light, as well as additional cleaning with heat and disinfectants.
Next steps include obtaining FDA approval for the kit, expanding its usage and conducting surveillance testing.
Other researchers in the group include Luke Buerer ’18, research technician Jing Wang, postdoctoral student Chaorui Duan, physician Gregory Jay, resident physician Caroline Meehan and clinical research assistant Sam Kaplan.
“It’s just nice to be able to help and work towards something,” Fairbrother said. “I feel really optimistic and sort of excited to go forward.”
ADVERTISEMENT