On Oct. 4, the Royal Swedish Academy of Sciences awarded the Nobel Prize in Chemistry to Massachusetts Institute of Technology Professor Moungi Bawendi, Columbia Professor Louis E. Brus and scientist Aleksey Yekimov “for the discovery and synthesis of quantum dots.”
For Ou Chen, associate professor of chemistry at Brown, the award hits very close to home: From 2010 to 2014, Chen worked as a postdoctoral assistant to Bawendi at MIT. Now, he researches quantum dots in his lab at Brown.
“The timing makes sense,” Chen said of the Nobel Prize. “Quantum dots have been (growing in prominence in) different fields, especially for display technology in the TV industry.”
“It’s been applied in this commercial product and is changing people’s lives, which is the definition of a Nobel Prize,” he added.
Lacie Dube GS, a researcher in Chen’s lab, hopes that Bawendi, Brus and Yekimov’s Nobel Prize win will draw more attention to the field of nanochemistry, which is the study of chemical phenomena and materials at the nanometer scale.
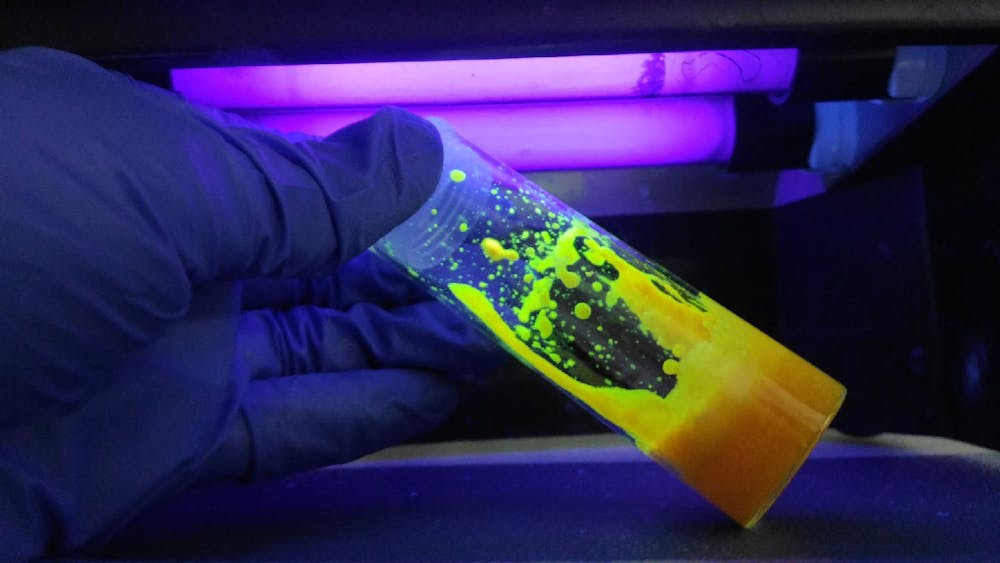
Quantum dots are artificially synthesized semiconductor nanocrystals — or, as Chen puts it, “tiny particles with huge potential.” But tiny may be an understatement: Quantum dots are “about as big to you as you are to the Sun,” according to Dube.
Quantum dots are made of materials that are neither insulators nor conductors, which gives them unique properties and capabilities. For example, the size of quantum dots, which are directly tied to the color of light they emit, can be manipulated during manufacturing, according to Chen. Typically, it is the material and composition of a particle that determines the color of light it emits.
“By changing the size of the particle,” Chen said, “you can totally transform the optical properties.”
Today, quantum dots have a diverse range of applications, from our TV screens to biomedical imaging techniques. Among the applications are laser display, biomedical sensing and photocatalysis, according to Shouheng Sun, professor of chemistry and engineering at Brown. In addition, quantum dots have been used for live imaging and drug delivery, further transforming medicine.
“All of these areas are from the fundamental work … initially developed” by Yekimov, Brus and Bawendi, Sun said.
Yekimov was the first to observe what physicists had previously theorized: Changing the size of a nanoparticle could change its optical properties. Yekimov showed this by changing the color of glass in the 1980s. A few years later, Brus independently showed similar results to Yekimov, this time in a fluid.
In 1993, Bawendi “revolutionized the chemical production of quantum dots, … (which was) necessary for them to be utilized,” according to a press release from the Royal Swedish Academy of Sciences.
At Brown, the Chen Lab is working on improving quantum dot synthesis, as well as studying the properties of the particles. The group seeks to develop a “variety of novel materials ranging from nanoscopic to macroscopic scales and characterizing and elucidating their chemical and physical properties for optical, energy, biological and catalysis applications,” according to their website.
“We’re trying to find new compositions, new kinds of materials and optimize their altered optical properties or make even more complex structures,” Chen said.
Dube is currently working on a project on perovskites, another class of nanomaterials closely related to quantum dots. Specifically, she is looking at lead-free alternatives for perovskite materials which “tend to be more stable and tend to have lower toxicity.”
Dube and Chen both stressed the young age of the nanochemistry field and are excited about its future. “A lot of new things have been discovered every year, every month,” Chen said. “It’s still very exciting.”
Dube said she is grateful to be exploring the field at this time: “There’s not many people that get to be at the start of something,” she said.

Ryan Doherty was the managing editor of digital content and vice president of The Herald's 135th editorial board. He is a junior from Carmel, NY who is concentrating in chemistry and economics. He previously served as a university news and science & research editor, covering faculty and higher education.