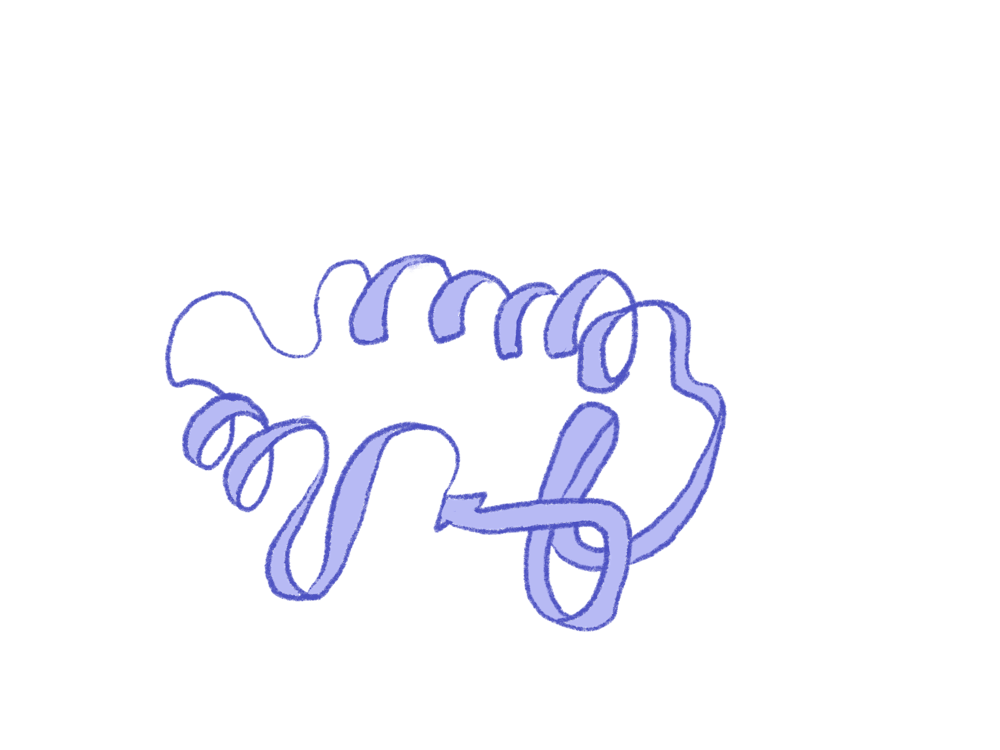University researchers developed a new technique to predict multiple protein conformations using an AI-based technology called AlphaFold2. Their findings, published in a March study, may aid in drug development.
Proteins are dynamic molecules that can shift between different folding conformations, said
Gabriel Monteiro Da Silva GS, a Ph.D. candidate and the paper’s first author. Historically, scientists have used time-intensive laboratory techniques to determine how a protein folds.
But those methods have limitations. “The problem with those methods is that they usually give you the structure of a protein at one point in time,” Monteiro Da Silva explained. But to fully understand a protein’s function, researchers must be aware of all of its different conformations.
George Lisi, assistant professor of molecular biology, cell biology and biochemistry, wrote in an email to The Herald that “these fleeting states can be detected experimentally, but atomic level snapshots are extremely difficult to capture, placing a premium on novel methods such as subsampled AlphaFold2.”
AlphaFold2 — developed by Google-affiliated company DeepMind — uses a model that has learned to predict protein structures based on the Protein Data Bank, an expansive database that Monteiro Da Silva described as “the dream of any person working with AI models.”
Usually, AlphaFold2 predicts the most commonly occurring structure, known as the ground state structure, according to Brenda Rubenstein, an associate professor of chemistry. But the team said that if they could repeatedly manipulate inputs, AlphaFold2 would output different conformations, represented proportionally to how they appear in cells.
“We didn’t develop AlphaFold2, but we came up with clever ways of using it,” she said, adding that this development allows scientists to “figure out all of that physics-based modeling in the space of hours as opposed to years.”
Understanding these changes in protein conformation can affect the efficacy of drugs that target those proteins. In the past, some drugs have been unsuccessful because “they were targeting one (protein) structure ... but not others,” Rubenstein said.
Rubenstein and Monteiro Da Silva explained that this technique works well for proteins that are well-studied because there is more data available to accurately train the model. The team was able to model kinases, a class of proteins linked to cancer that have historically been difficult to target because of their dynamic nature.
But the method is not as accurate for proteins with less preexisting data available, Rubenstein added.
Often “the output shape is broadly correct, but when you look (at) the grain, you might see some imperfections,” Monteiro Da Silva said. “Those imperfections will impact the use of these particular models for some very specific things.”
Since these granular errors may impact drug development, the team is working to improve the technology’s accuracy.
In addition to helping improve drugs, “we are looking at this as a way to predict the dynamics and movements of the proteins,” Rubenstein said. “If we can predict multiple structures, now maybe we can also predict the movements going between those,” which has additional implications for drug development.
Monteiro Da Silva also looks to make the technique more accessible. “We want to make it something that anyone who has access to a computer terminal and some basic coding skills can do,” he said.

Francesca Grossberg is a staff writer covering Science and Research. She is a sophomore from New York City studying Health and Human Biology.